Background
Randomized clinical trials (RCTs) are crucial for medical advancements but often need more participant diversity, distorting our understanding of whether such advancements are effective across all populations. The lack of diverse representation of participants in RCTs can also perpetuate disparities in care. This study examines disparities in leukemia trials, underscoring the need to address equity in RCT representation.
Methods:
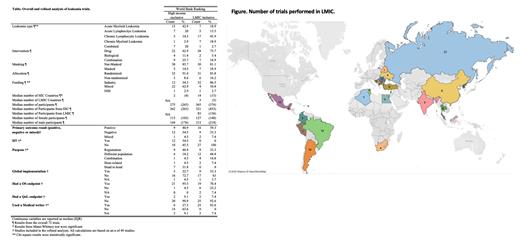
We analyzed phase 3 acute and chronic leukemia RCTs listed on ClinicalTrials.gov from January 2012 to March 2023. We selected these trials due to their potential to offer insights into the effectiveness and adverse effects of treatments. Our primary objective was to delineate disparities in trial demographics and characteristics, with a distinct focus on participant recruitment regions in relation to global leukemia incidence and prevalence.
Two independent team members used a standardized form to extract study and participant demographic characteristics, treatment type, and outcomes data and classified participant countries based on the World Bank Ranking (WBR). The recruitment countries and participant numbers were gathered from the clinicaltrials.gov registry and further classified by the WBR into HIC-exclusive and LMIC-inclusive. Initially, we performed a descriptive analysis of all trials to delve into the characteristics of the RCTs. To measure completion rates, we divided the number of participants who finished by the number of participants who started.
The second phase of our analysis was a more in-depth evaluation of published trials. We cross-referenced the registry for the corresponding publication. If the research paper was not found after an online search by two authors, it was excluded from the refined analysis. We collected information on the primary author (PA) and the number of authors affiliated with HIC and LMIC institutions. Additionally, we extracted the journal impact factor (JIF) from the year the paper was published.
Results:
Our analysis of 72 trials and 27,382 participants revealed an overrepresentation of participants from HICs (90.4%) compared to LMICs (9.6%). Most of the studies were drug-based (69.4%) and open-label (83.3%), focusing mainly on acute myelogenous leukemia (AML) and chronic lymphocytic leukemia (CLL). More males than females participated, with a median ratio of 1.44. Completion rates varied widely from 0% to 100% (median: 69%). A significant portion of these trials (61.1%) were industry-funded, and approximately 70% were published. Interestingly, some trials (12.5%) were non-randomized, despite being registered as phase 3.
Of the 72 trials, 35 were exclusively from HICs, favoring AML, while LMIC-inclusive trials were more leaned towards CLL. The median number of LMICs per trial was considerably low (3 countries) compared to HICs (14 countries). The median number of total participants was lower in HIC-exclusive (275) compared to LMIC-inclusive (365) trials. This applied to both male and female patients: HIC-exclusive: 115 females, 144 males; LMIC-inclusive 127 females, 215 males.
Only 3 trials exclusively involved LMIC participants, all of which were from China and focused on CML. These trials were non-randomized, industry-funded, and drug-focused. Two of these trials reported positive results, with one discussing global implementation, but none reported quality-of-life (QoL) outcomes.
Of the 49 studies with published research, only a few studies (28%) discussed global implementation and considered QoL endpoints (8%). LMIC-inclusive trials reported more outcomes with positive connotations (60%) and frequently employed medical writers (93%). None of the LMIC-inclusive trials were investigator-initiated. The US was the predominant FA country. China was the only LMIC with a local FA country (n=3). Most authors came from HIC institutions. We found significant differences between HICs and LMICs, and moderate negative correlations between the number of LMIC authors and JIF.
Conclusion:
Our findings highlight a pressing need to improve LMIC representation in leukemia RCTs. Aligning trial demographics with global leukemia prevalence is vital since more than 80% of the global leukemia population resides in LMICs. Inclusive and diverse trial demographics are essential not just for statistical balance but for addressing enduring health disparities in underrepresented regions.
Disclosures
Cortes:Takeda: Consultancy, Honoraria; Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Gomez-Almaguer:AMGEN: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Honoraria; AbbVie: Consultancy, Honoraria. Gomez-De Leon:AMGEN: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Jnssen: Other: Advisory board; Sanofi: Honoraria.